A Cosmetic Tattooing Procedure to Conceal Hair Loss
Scalp Micropigmentation, the process of tattooing thinning or bald areas of the scalp to resemble shortly cropped hair, has been met with high amounts of enthusiasm from patients seeking alternative ways to mask and conceal their hair loss. This artistic and minimally invasive procedure, also referred to as SMP, provides men and women suffering from hair loss with an illusion of fullness or the appearance of thick, shortly cropped hair. SMP results (not the procedure) are often compared to topical concealers such as Toppik, DermMatch and Nanogen, because they also help conceal baldness and make thinning hair appear thicker and fuller.
Although scalp micropigmentation is increasing in popularity, it is still a new and evolving practice and its mystique may leave interested patients asking several important questions.
How does scalp micropigmentation work? What are the benefits, limitations and potential risks associated with the procedure? Who is a suitable candidate? What are the costs associated with SMP? What happens if consumers don't like the results? Is the ink associated with scalp micropigmentation permanent? Are all SMP practitioners equal or do some produce better, more natural looking results?
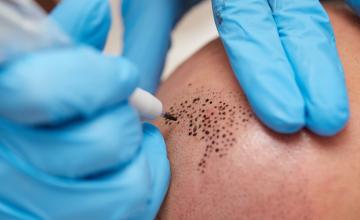
Scalp Micropigmentation is the artistic application of tattoo-like ink or pigmentation "dots" to bald or thinning areas of the scalp to recreate the appearance of shortly cropped hair (or "stubble") by a trained practitioner. SMP practitioners are typically experienced in the art but often aren't medical professionals or hair restoration physicians. Scalp micropigmentation is typically used for one of three purposes. These include:
- Creating the illusion of fullness or density to thinning hair by applying cropped, hair-like tattoos in between and around thinning hair.
- Creating the appearance of closely cropped or shaved hair on an otherwise bald scalp.
- Camouflaging a pre-existing hair transplant scar. Typically, this is a rare, but stretched, linear scars from follicular unit hair transplantation procedures via strip harvesting (FUT) can sometimes require revision.
Scalp micropigmentation ink is normally placed in the superficial dermis, a layer of skin between the epidermis (the outer layer of the skin) and subcutaneous tissues that consists of connective tissue and cushions the body from stress and strain. However, SMP practitioners often place ink at varying depths. Some practitioners are now beginning to use a “sweeping” technique when tattooing, in order to create a more three-dimensional appearance on the scalp.
Depending on the size of the balding/thinning area and the desired appearance, the procedure can take anywhere from 1-8 hours, and can now be completed with both permanent and temporary SMP ink.
According to Coalition hair transplant surgeon Dr. Robert Bernstein, the key to further achieving a natural result with SMP lies in holding the inking tool at an appropriate angle, controlling the depth at which the ink is placed, monitoring the amount of ink deposited at each site, and selecting appropriate ink tones.
To read more of Dr. Bernstein's input and discussion related to SMP at the 2012 annual ISHRS Scientific Meeting for hair restoration physicians, visit "Scalp Micropigmentation (Scalp Tattooing)at the 2012 ISHRS (International Society of Hair Restoration Surgery) Scientific Meeting".
Permanent Versus Temporary Scalp Micropigmentation (Advantages and Disadvantages)
Defining and understanding the difference between permanent and temporary ink is important for any consumer considering the procedure.
Permanent scalp micropigmentation is performed with micropigmentation ink that is not designed to fade or change over time. The ink is placed slightly deeper in the scalp tissue, and the procedure can be performed with ink containing both colored and black pigment.
Advantages of permanent SMP include the ability to undergo a single SMP treatment without frequent “touch ups" and potentially lower long-term costs.
Disadvantages include the possibility that the ink will eventually fade to a green or blue hue (due mainly to the black pigment used in permanent SMP ink) and the inability to reverse the procedure without laser pigment removal (i.e. “laser tattoo removal”) if the work is unsatisfactory.
Temporary scalp micropigmentation is performed with ink that is specially designed to gradually fade and disappear within 6 to 24 months after the procedure. The ink is placed into a superficial layer of the scalp, which allegedly helps to define the pigmentation and decrease the probability of “blurring” ink. Temporary SMP is performed with ink containing no black pigment. Because black pigmentation can eventually fade into a blue or green tone, it would inevitably produce an unnatural looking result as it gradually fades and disappears, and should be avoided.
Advantages of temporary SMP include: the ability to redesign or discontinue the process (when the ink fades after 6-24 months) if the results are not satisfactory; the decreased rates of ink blurring, running, and definition loss with the superficial placement into the scalp; and the ability to use the three-dimension technique (which was designed by Beauty Medical in Milan, Italy, and is now utilized by several leading clinics recommended by this patient community).
Disadvantages of temporary SMP include ongoing maintenance and costs associated with undergoing subsequent SMP applications every 6 to 24 months, and the possibility of discoloration as the ink fades (though the temporary ink is purposely designed to fade evenly and without discoloration).
To learn more about the advantages and disadvantages of permanent and temporary SMP, visit "Temporary Scalp Micropigmentation: Advantages, Disadvantages, and Clinics Currently Offering the Procedure".
Scalp Micropigmentation Costs
The cost of SMP is variable and depends upon the type of procedure (temporary versus permanent), size of the procedure, and the practitioner/clinic performing the micropigmentation. While rates typically vary and change, permanent SMP can cost anywhere from $800 for a small procedure and $6000 for a large one. The cost of temporary SMP is approximately half the cost of permanent SMP and typically ranges from $500 to $2500 depending on the size of the procedure. Subsequent procedures, which are needed 6 to 24 months after the initial results fade are typically 50% of the original costs.
What Makes a Good SMP Candidate?
Although a wide variety of men and women are interested in scalp micropigmentation, the procedure is actually only ideally suited for a small, select number of hair loss consumers.
SMP is likely best suited for individuals interested in aiding the appearance of density in shortly cropped, diffusely thinned hair, and for patients trying to camouflage a hair transplant scar. It may also be suitable in creating a greater illusion of fullness in those who've already undergone surgical hair restoration.
SMP consumers should also consider a naturally asymmetrical hairline design and “fading” effect (from decreased pigmentation in the hairline to denser, increased pigmentation in the middle scalp) if hairline restoration is performed. This approach will likely create a more natural result in eligible candidates.
Creating Realistic Expectations
While scalp micropigmentation may be a useful adjunct therapy for a select group of consumers interested in concealing their hair loss, it should be approached with realistic expectations and performed by a trusted clinic. Individuals considering the procedure must remember that SMP is a two-dimensional process and cannot provide the texture and growth of real hair. Undergoing SMP without respecting its limitations may create unrealistic expectations and disappointing results.
Evolving Procedure or Just a Fad?
Is SMP just a fad or will it continue to evolve and cultivate additional interest from hair loss sufferers and hair restoration physicians?
While some hair loss experts are quickly embracing the procedure, others are more resistant, and liken scalp micropigmentation to less popular "niche" treatments like hair systems, laser caps, and topical concealers. Whether or not it will continue increasing in popularity and retain its momentum is not yet clear.
Hair restoration is a rapidly changing field and new and evolving therapies may eventually render scalp micropigmentation and other hair loss treatments obsolete. Altogether, only time will tell whether SMP is a lasting adjunct therapy or a passing fad.
Clinics Currently Offering Scalp Micropigmentation
As of January 2013, the following hair restoration clinics recommended by this website are offering some form of Scalp Micropigmentation: Hasson and Wong (Drs. Victor Hasson and Jerry Wong), and Shapiro Medical Group (Drs. Ron and Paul Shapiro).
Dr. Feller, Dr. Lindsey, Hasson and Wong, and Shapiro Medical Group trained with Beauty Medical and offer the temporary SMP procedure; Dr. William Rassman performs his own variation of Scalp Micropigmentation with permanent ink.
Note that in many cases, a trained technician and not the physician will be performing scalp micropigmentation.
This website does not currently recommend one SMP clinic over another. Those considering scalp micropigmentation as a tool to conceal hair loss and create an illusion of hair are encouraged to do their own diligence in researching each technique, practitioner and clinic.
To discuss scalp micropigmentation with hair loss sufferers and other interested parties and to view results showing before and after pictures, visit the Scalp Micropigmentation Forum.







 Crinagen is an all natural alcohol-free topical scalp spray clinically backed to reduce the amount of DHT (dehydrotestosterone) in the scalp. It contains no alcohol, and has displayed no side effects. Crinagen is also equally safe for men and women and is most effective in men and
Crinagen is an all natural alcohol-free topical scalp spray clinically backed to reduce the amount of DHT (dehydrotestosterone) in the scalp. It contains no alcohol, and has displayed no side effects. Crinagen is also equally safe for men and women and is most effective in men and 

 A Viable Alternative to Propecia in Treating Hair Loss?
A Viable Alternative to Propecia in Treating Hair Loss?